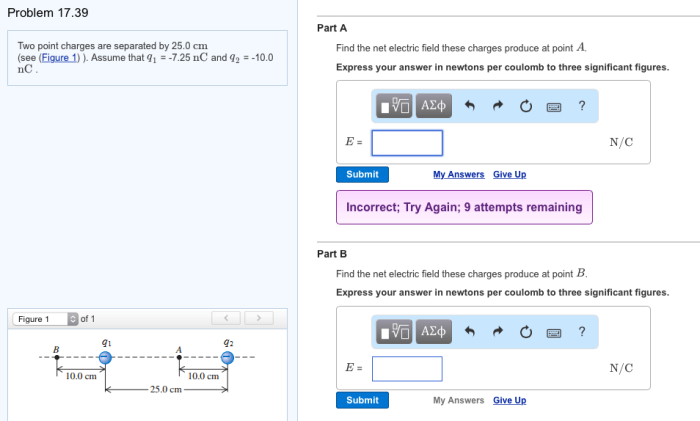
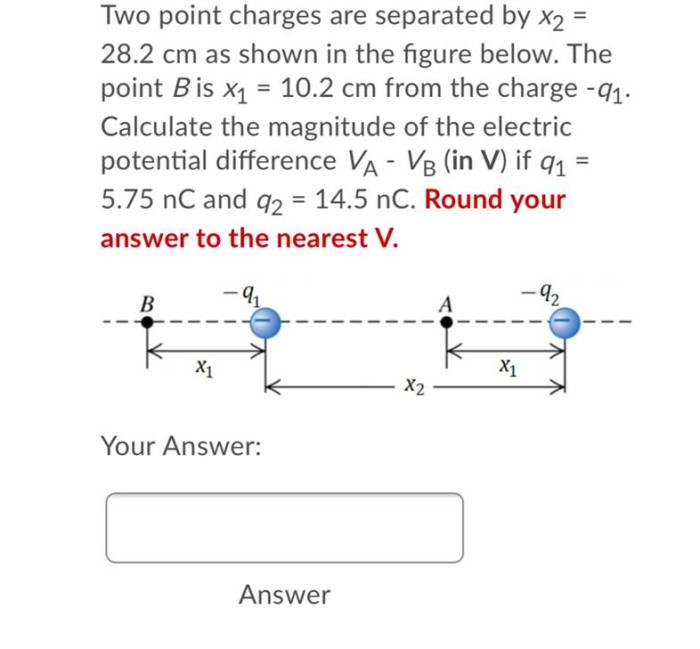
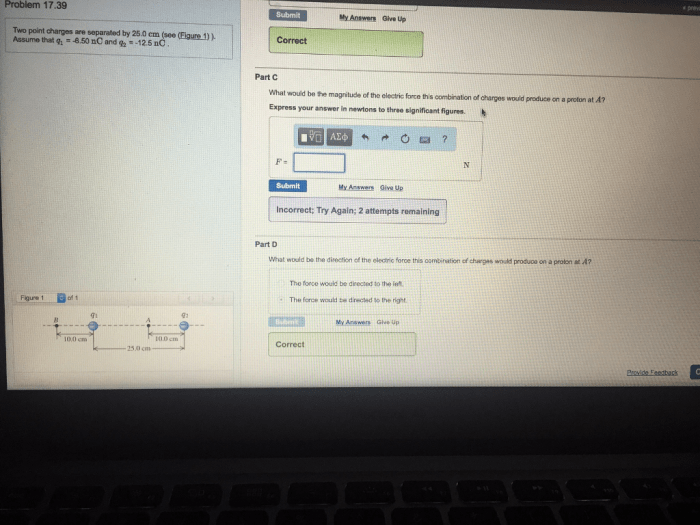
Two point charges are separated by 25.0 cm – Two point charges separated by 25.0 cm embark on an electrostatic adventure, governed by the enigmatic Coulomb’s Law. This fundamental principle unveils the intricate dance of forces between charged particles, shaping our understanding of electromagnetism.
Delving into the heart of Coulomb’s Law, we unravel its mathematical elegance and explore its profound implications for electrostatic interactions. We embark on a journey to decipher the factors that modulate the force between charges, unraveling the interplay between charge magnitude, distance, and the intervening medium.
Coulomb’s Law
Coulomb’s Law is a fundamental principle in electrostatics that describes the force between two point charges.
Define and Explain Coulomb’s Law
Coulomb’s Law states that the force between two point charges is directly proportional to the product of the charges and inversely proportional to the square of the distance between them.
Calculate the Force Between Two Point Charges
To calculate the force between two point charges, use the formula:
F = k
- (q1
- q2) / r^2
where:
- F is the force between the charges
- k is Coulomb’s constant (8.98755 x 10^9 N m^2/C^2)
- q1 and q2 are the charges of the two point charges
- r is the distance between the charges
Factors Affecting the Force Between Two Point Charges, Two point charges are separated by 25.0 cm
The force between two point charges is influenced by several factors:
- Magnitude of the charges:The force is directly proportional to the product of the charges.
- Distance between the charges:The force is inversely proportional to the square of the distance between the charges.
- Medium between the charges:The presence of a medium (e.g., air, water) can affect the force.
Applications of Coulomb’s Law
Coulomb’s Law has numerous applications in various fields, including:
- Electrical circuits:Designing circuits to control the flow of electricity.
- Electrostatic phenomena:Understanding the behavior of charged objects and electrostatic interactions.
- Electrochemistry:Investigating electrochemical reactions and processes.
FAQ Summary: Two Point Charges Are Separated By 25.0 Cm
What is the significance of Coulomb’s Law?
Coulomb’s Law quantifies the electrostatic force between charged particles, providing a fundamental understanding of their interactions and the behavior of matter at the atomic and molecular level.
How does the distance between charges affect the electrostatic force?
The electrostatic force between charges decreases rapidly with increasing distance, following an inverse square law relationship. Doubling the distance between charges reduces the force by a factor of four.
What is the role of the medium between charges in Coulomb’s Law?
The medium between charges can influence the electrostatic force. For example, the presence of a dielectric material reduces the force compared to a vacuum.