Embark on a captivating journey into the realm of dehydration synthesis and hydrolysis practice, where chemical reactions unfold with precision and elegance. This comprehensive guide unravels the intricacies of these fundamental processes, providing a thorough understanding through engaging examples, insightful explanations, and practical problem-solving exercises.
Delve into the mechanisms of dehydration synthesis, witnessing the removal of water molecules to form new covalent bonds. Explore the diverse applications of hydrolysis, from breaking down complex molecules to fueling cellular processes. Prepare to master the art of reaction prediction and problem-solving through a series of expertly crafted practice problems.
Dehydration Synthesis
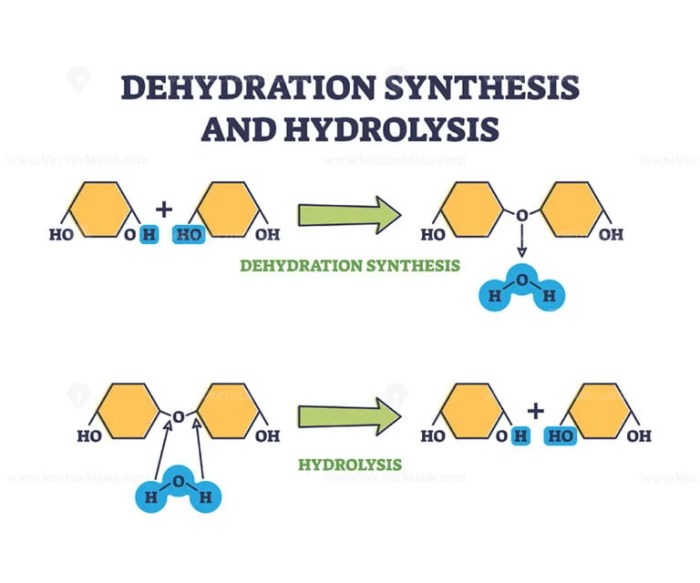
Dehydration synthesis is a chemical reaction in which two molecules combine to form a larger molecule, with the removal of a water molecule. It is a type of condensation reaction.
The general equation for dehydration synthesis is:
A-OH + B-H → A-B + H 2O
where A and B are functional groups.
Dehydration synthesis reactions are typically catalyzed by enzymes. Enzymes are proteins that speed up the rate of chemical reactions without being consumed in the reaction.
Examples of Dehydration Synthesis Reactions
- The formation of a peptide bond between two amino acids
- The formation of a glycosidic bond between two monosaccharides
- The formation of an ester bond between an alcohol and a carboxylic acid
Hydrolysis
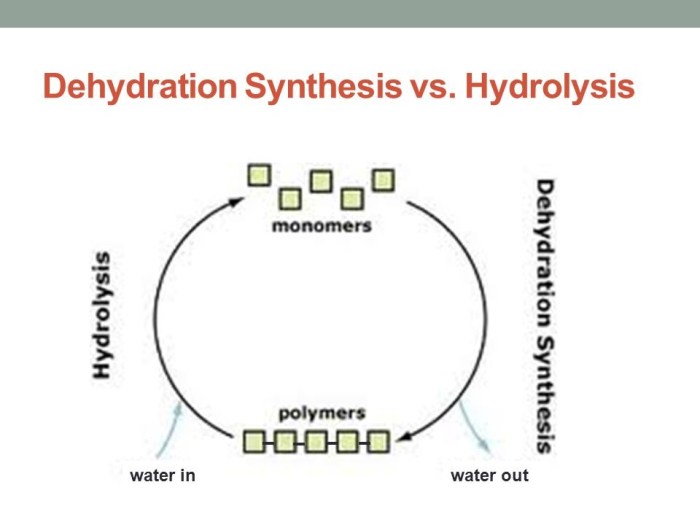
Hydrolysis is a chemical reaction in which a molecule is broken down into smaller molecules by the addition of water. It is the opposite of dehydration synthesis.
The general equation for hydrolysis is:
A-B + H 2O → A-OH + B-H
where A and B are functional groups.
Hydrolysis reactions are typically catalyzed by enzymes. Enzymes are proteins that speed up the rate of chemical reactions without being consumed in the reaction.
Examples of Hydrolysis Reactions
- The breakdown of a peptide bond between two amino acids
- The breakdown of a glycosidic bond between two monosaccharides
- The breakdown of an ester bond between an alcohol and a carboxylic acid
Comparison of Dehydration Synthesis and Hydrolysis: Dehydration Synthesis And Hydrolysis Practice
| Reaction Type | Reactants | Products | Energy Change |
|---|---|---|---|
| Dehydration Synthesis | Two molecules with functional groups | One larger molecule and water | Endergonic (requires energy) |
| Hydrolysis | One molecule with a functional group and water | Two smaller molecules | Exergonic (releases energy) |
Practice Problems

Problem 1
Write the equation for the dehydration synthesis reaction between glucose and fructose to form sucrose.
Solution, Dehydration synthesis and hydrolysis practice
The equation for the dehydration synthesis reaction between glucose and fructose to form sucrose is:
C 6H 12O 6+ C 6H 12O 6→ C 12H 22O 11+ H 2O
Problem 2
Predict the products of the hydrolysis reaction of starch.
Solution, Dehydration synthesis and hydrolysis practice
The products of the hydrolysis reaction of starch are glucose molecules.
Query Resolution
What is the key difference between dehydration synthesis and hydrolysis?
Dehydration synthesis involves the removal of water molecules to form new bonds, while hydrolysis involves the addition of water molecules to break bonds.
What are some common examples of dehydration synthesis reactions?
Dehydration synthesis reactions include the formation of esters, amides, and ethers.
How do enzymes play a role in hydrolysis reactions?
Enzymes act as catalysts, increasing the rate of hydrolysis reactions by lowering the activation energy.